luohuichao@csmspharm.com
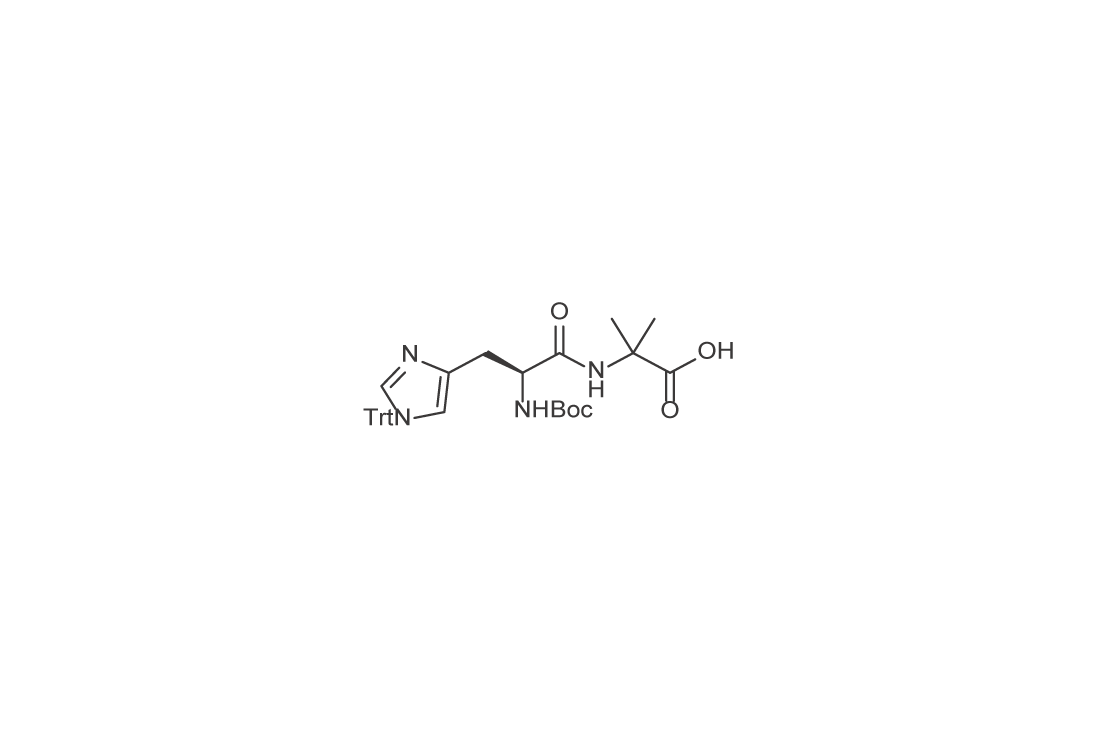
Used as a starting material for the synthesis of semaglutide, this product inserts histidine and modified amino acids (alanine → α-aminoisobutyric acid, resistant to DPP-4 degradation) at the 7th and 8th positions of semaglutide, respectively.
Excellent Morphological Properties: white or kind of white powder.\nHigh Purity and Content: individual impurity < 0.10%, including the isomeric impurity < 0.10%.\nGMP-Compliant Manufacturing: manufactured and released by an GMP-certified API facility. Large production capacity, exceeding 100 kilograms per batch.
Comprehensive Quality Studies: able to perform comprehensive quality studies according to the client's requirements, including related substances, residual solvents, genotoxic impurities, inorganic impurities, structure identification, and stability testing, etc. All the methods are well validated and can be transferred to clients. The related impurity references can also be provided.